Tubeufiaceae
Clement Tsui and Mary Berbee

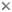
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxThis tree illustrates the phylogenetic relationships among the genera within the family based on the analyses of rDNA requences
Introduction
The family Tubeufiaceae contains fungi producing fleshy, white, bright-colored to black ascomata, and clavate to cylindrical bitunicate asci, and mostly hyaline, fusiform to fusoid, and multi-septate ascospores (Rossman, 1987). It was established to accommodate bitunicate ascomycetes which superficially resemble members of the Hypocreales (Barr, 1980; Rossman, 1987). The family has ca. 23 genera and over 80 species. Most taxa are parasitic to fungi and plants, or some are saprobic. The family also has anamorphic fungi that produce beautiful helicospores or long phragmospores (Goos, 1985, 1986, 1989; Tsui et al. in prep). Many helicosporous fungi are considered aero-aquatic fungi in ecology because they occur commonly in ponds or streams but sporulate only when exposed to air (Webster and Descals, 1981). In some species, the spores are buoyant and can trap air within for efficient dispersal (Webster and Descals, 1981).


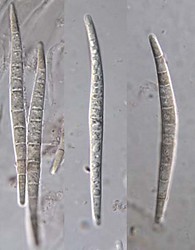
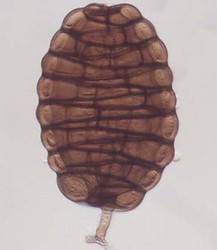
Ascomata (left) and ascospores (right) of Tubeufia cylindrothecia. © Amy Y. Rossman.
Ribosomal DNA placed the family within the Dothideomycetes. The Tubeufiaceae s. str. was designated since six Tubeufia species sensu Barr (1980), including Tubeufia paludosa, a synonym of T. helicomyces (the type species of Tubeufia), together with many helicosporous anamorphs formed a strong, monophyletic clade within the Dothideomycetes with 100% bootstrap support (Tsui and Berbee, 2006). However in the same study, molecular evidence also confirmed convergent evolution of spore form in other ascomycete families. This may represent adaptation to dispersal in aquatic environments.
The following list of genera belonging to Tubeufiaceae according to Myconet shows that only a small proportion of the family has been investigated with molecular data: * genera have been included in the phylogenetic studies.
- Acanthostigma*
- Acanthophiobolus
- Acanthostigmella*
- Allonecte
- Amphinectria
- Boerlagiomyces*
- Byssocallis
- Chaetocrea
- Chaetosphaerulina
- Glaxoa
- Letendraea*
- Letendraeopsis
- Malacaria
- Melioliphila
- Paranectriella
- Podonectria
- Puttemansia
- Rebentischia
- Taphrophila*
- Thaxteriellopsis
- Thaxterina
- Tubeufia*
- Uredinophila
Despite the monophyly of Tubeufia sensu Barr, the phylogenetic positions of other genera in the family are challenged. Letendraea was included in Tubeufiaceae as it produces thin-walled, pallid ascomata and one-septate ascospores (Barr, 1980). Repeated molecular data confirm the phylogenetic placement of Letendraea helminthicola in the Pleosporales (Lumbsch and Lindemuth, 2001, Kodsueb et al. 2006). Boerlagiomyces, though producing hyaline, muriform ascospores, was included in the Tubeufiaceae because of its membranous, brown, setose ascomata (Crane et al. 1998). Recently B. websteri was shown to group with Rhytisma acerinum in the Pezizomycotina, and it possibly should be excluded from the Tubeufiaceae (Kodsueb et al. 2006). Acanthostigmella brevispina has affinity to Tubeufia because of its ascomata and asci morphology and the presence of pseudoparaphyses (Crane et al., 1998). But molecular evidence showed that the taxon originated from Pleosporales (Untereiner et al., 1995).
Discussion of Phylogenetic Relationships
Within the Tubeufiaceae s. str., the teleomorphic genera Acanthostigma, Thaxteriella and Tubeufia are distinguished primarily on ascomata morphology (Barr 1980, Rossman 1987, Crane et al 1998). Thaxteriella produces brown to black ascomata, and the type species, T. pezizula, is linked to a Helicoma anamorph (Pirozynski 1972), while Tubeufia species are white to yellow, often brightly colored, and the type produces a Helicosporium anamorph (Barr 1980, Goos 1989). Acanthostigma species can have either Helicomyces or Helicosporium anamorphs but unlike the other genera, their ascomata have setae (Réblová and Barr 2000, Kodsueb et al 2004). These generic concepts are confusing (Pirozynski 1972, Crane et al 1998, Réblová and Barr 2000), and Thaxteriella is variously retained as a distinct genus or made a section within Tubeufia.
Phylogenetic analyses of sequences from ITS, 5.8S and LSU rDNA did not support the monophyletic groupings of Thaxteriella and Tubeufia spp. and cast doubt on the separation of the two genera. Also the relationship of Acanthostigma to Tubeufia is uncertain because Tubeufia cerea clustered with Acanthostigma perpusillum, which is the type species of the genus. Probably Thaxteriella and Acanthostigma should be included within an expanded concept of Tubeufia.
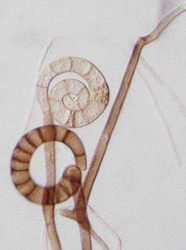
The Tubuefiaceae contains many helicosporous anamorphs belonging to genera Helicoma, Helicomyces or Helicosporium (Goos 1985, 1986, 1989). They have traditionally been differentiated by the morphology of their conidia and conidiophores. Ribosomal sequences from ITS, 5.8S and partial LSU regions differentiated 45 isolates into seven clades that each received 79% or more bootstrap support (Tsui et al. 2006). However, none of the genera were monophyletic. The 15 isolates of Helicoma were scattered throughout the phylogeny and appeared in five of the clades. The Helicosporium species also appeared in five clades. The four Helicomyces species were distributed among three clades. Traditional characters such as the thickness of the conidial filament and whether conidiophores were conspicuous or reduced proved to be poor predictors of phylogenetic relationships. Most of the clades supported by sequence data lacked unifying morphological characters.
Current results show that the taxonomic positions of Tubeufia and their anamorphic fungi remain unclear, additional specimens and genes, and further molecular analysis are needed to resolve the monophyly of the genus and family.
References
Barr ME. 1980. On the family Tubeufiaceae. Mycotaxon 12:137-167.
Crane JL, Shearer CA, Barr ME. 1998. A revision of Boerlagiomyces with notes and a key to the saprobic genera of Tubeufiaceae. Canadian Journal of Botany 76:602-612.
Goos RD. 1985. A review of the anamorph genus Helicomyces. Mycologia 77:606-618.
Goos RD. 1986. A review of the anamorph genus Helicoma. Mycologia 78:744-761.
Goos RD. 1989. On the anamorph genera Helicosporium and Drepanospora. Mycologia 81:356-374.
Kodsueb R, Lumyong S, Lumyong P, McKenzie EHC, Ho WH, Hyde KD. 2004. Acanthostigma and Tubeufia species, including T. claspisphaeria sp. nov., from submerged wood in Hong Kong. Mycologia 96:667-674.
Kodsueb R, Jeewon R, Dhanasekaren V, McKenzie EHC, Lumyong P, Lumyong S, Hyde KD. 2006. Systematic revision of Tubeufiaceae based on morphological and molecular data. Fungal Diversity 21:105-130.
Lumbsch HT, Lindemuth R. 2001. Major lineages of Dothideomycetes (ascomycetes) inferred from SSU and LSU rDNA sequences. Mycological Research 105: 901-908.
Pirozynski KA. 1972. Micro Fungi of Tanzania. I. Miscellaneous fungi on oil palm. Mycological Papers 129: 1-39.
Réblová M, Barr ME. 2000. The genus Acanthostigma (Tubeufiaceae, Pleosporales). Sydowia 52:258-285.
Rossman AY. 1987. The Tubeufiaceae and similar loculoascomycetes. Mycological Papers 157:1-71.
Tsui CKM, Berbee ML. 2006. Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Molecular Phylogenetics and Evolution 39: 587-597.
Tsui CKM, Sivichai S, Berbee ML. 2006. Molecular systematics of Helicoma, Helicomyces and Helicosporium and their teleomorphs inferred from rDNA. Mycologia 98: 100-110.
Tsui CKM, Sivichai S, Rossman AR, Berbee ML. 2006. Aquaphila has phylogenetic affinity in Tubeufiaceae based on rDNA sequences. Canadian Journal of Botany (in prep)
Untereiner WA, Straus NA, Mallach D. 1995. A molecular-morphotaxonomic approach to the systematics of the Herpotrichiellaceae and allied black yeasts. Mycological Research 99: 897-913.
Webster J, Descals E. 1981. Morphology, distribution and ecology of conidial fungi in freshwater habitats. In: Cole GT, Kendrick B. (Eds.), Biology of conidial fungi. Academic Press, New York pp. 295-355.
Title Illustrations

| Scientific Name | Helicoon gigantisporum |
|---|---|
| Reference | Molecular Phylogenetics and Evolution 39:587-597 (2006) |
| Acknowledgements | courtesy Dr. Somsak Sivichai |
| Specimen Condition | Live Specimen |
| Identified By | Somsak Sivichai |
| Body Part | Conidia |
| Copyright | © Dr. Somsak Sivichai |
| Scientific Name | Helicoma pulchra |
|---|---|
| Acknowledgements | Electron Microscopy Unit at UBC |
| Specimen Condition | Culture |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
©

|
| Scientific Name | Helicoma violaceum |
|---|---|
| Reference | Molecular Phylogenetics and Evolution 39:587-597 (2006) |
| Acknowledgements | Electron Microscopy Unit at UBC |
| Specimen Condition | culture |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
©

|
About This Page
We are grateful to BCC, CBS and MUCL for cultures; Seara Lim, Patrik Inderbitzin, Somsak Sivichai, Amy Rossman, Keith Seifert for scientific advice and techinical assitance. The Croucher Foundation and NSERC are thanked for financial support.

University of British Columbia, Vancouver, British Columbia, Canada
Mary Berbee

University of British Columbia, Vancouver, British Columbia, Canada
Correspondence regarding this page should be directed to Clement Tsui at
Page copyright © 2007 and Mary Berbee
 Page: Tree of Life
Tubeufiaceae.
Authored by
Clement Tsui and Mary Berbee.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Tubeufiaceae.
Authored by
Clement Tsui and Mary Berbee.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 19 March 2007
- Content changed 19 March 2007
Citing this page:
Tsui, Clement and Mary Berbee. 2007. Tubeufiaceae. Version 19 March 2007 (under construction). http://tolweb.org/Tubeufiaceae/60229/2007.03.19 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site