Acantharia
Demetrio Boltovskoy and Nancy M. Correa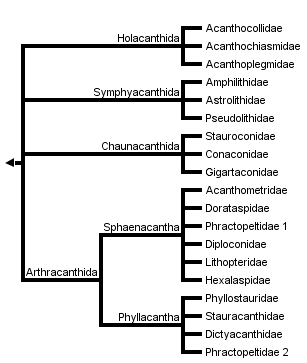

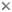
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Acantharia are planktonic, free living, exclusively marine protozoa, ranging in size from 0.05-5 mm in diameter. Like several other marine protists (Radiolaria, Heliozoa), acantharians have axopodia: long, radiating processes used for capturing prey (Fig. 1). Their distinguishing features are: (1) a skeleton of strontium sulfate (SrSO4), or celestite, based on a general plan of 10 diametral or 20 radial spicules arranged in a regular pattern called Müller's law (Müller, 1859; Fig. 2, 3); (2) cell body covered with an outer pellicle, the periplasmic cortex (Fig. 4); (3) the myonemes, contractile filaments grouped around the spicules (Fig. 4).
There is no direct evidence of the origin or geologic history of the Acantharia because their skeletons do not preserve in the sediments. In fact, the extreme solubility of the celestite skeletons of acantharians is one of the reasons for the scarcity of information on this group. Solubility prevents their retention in sea water samples preserved by conventional methods, making the addition of strontium to preserved samples necessary.


Fig. 1. Astrolithium sp. courtesy micro*scope © David Patterson, Linda Amaral Zettler, Mike Peglar and Tom Nerad
Acantharia were formerly grouped together with the Polycystina and the Phaeodaria under the common name Radiolaria, but recent molecular evidences suggest that they have not evolved from the same lineage as the Phaeodaria, yet their relationships with the Polycystina are still controversial (Amaral Zettler et al., 1997; López-García et al., 2002; Polet et al., 2004). Furthermore, recent molecular studies of mesopelagic materials off California suggest the existence of acantharian sequences, but the morphology of these organisms is unknown (Gilga et al., 2010).
Characteristics
Morphology
The cell consists of a central endoplasm and a peripheral ectoplasm, separated by a membrane - the central capsule. The endoplasm, which contains the nuclei, mitochondria, ribosomes, Golgi, endoplasmic reticulum, dictyosomes and various other organelles, is often brown, red, or black. The ectoplasm contains the digestive vacuoles. The cell is provided with axopodia, slender processes radiating from the cell surface which are sensitive and can retract upon chemical or physical stimulation. They are stiffened by a central axis (axoneme). The cytoplasm around each spicule contains 2-60 fibrillar myonemes anchored by one end to the spicule and by the other end to the periplasmic cortex (Fig. 4). The myonemes are motor organelles capable of active movement which govern the size of the cell and, probably, play a part in buoyancy regulation.

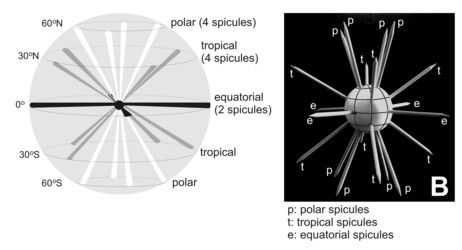
Fig. 2. Scheme of the spatial distribution of acantharian spicules according to Müller´s law. From Bernstein et al. (1999). © .
The acantharian skeleton, composed of strontium sulfate, consists of 10 diametral (or 20 radial), solid spicules which cross the cytoplasm. The distal ends of the spicules arise at precise points on the cell surface, which Müller (1859) described in an analogy to the surface of the earth (Fig. 2). This distribution of spicules is a general rule in the group, but differences in their degree of development (Fig. 3) are responsible for a vast variety of shapes. While in many species spicules are of equal length, in several families 2 or 4 equatorial spicules are larger and/or different in shape from the others, and either similar or different among themselves in size and shape. Differences can also be found between polar and tropical spicules, and can in turn lead to marked changes in the overall body shape: spherical, ovoid, lenticular, etc. The shape of the spicules is highly variable (Fig. 3), from simple cylindrical rods, to elaborate 4- or 6-bladed structures with smooth or serrated edges.

Fig. 3. Different types of acantharian spicules. From Haeckel (1887) and Reshetnjak (1981).
In some species the spicules cross loosely in the center of the cell, but in others they fuse in the center into a sphere or a star-like mass. Many acantharians develop a complementary meshwork and/or plates forming a shell. These additional structures are derived from anastomosing and merger of lateral apophyses of the spicules. Thus, each spicule can generate a pentagonal or hexagonal plate by lateral growth. Some species have 2 (rather than 1) concentric spheres, others have large wing-like meshwork apophyses on the distal ends of the main spicules, or large opposite conical "sleeves", etc. The overall size, aspect, and complexity of the skeleton can change with growth and geographic area.

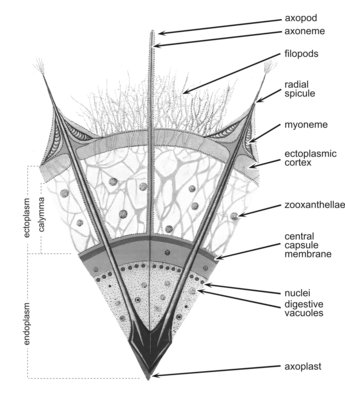
Fig. 4. Cross section of an acantharian cell. From Reshetnjak (1981).
Reproduction
Reproduction and the life cycle are incompletely known. There is no evidence for asexual reproduction, although it was previously reported in one genus (Schewiakoff, 1926). Sexual reproduction is undertaken by a gamont, morphologically undistinguishable from a vegetative cell, or through the formation of a cyst. Encystment involves a complete reorganization of the mineral skeleton and a series of mitotic and meiotic fissions which yield thousands of biflagellate isogametes.
Feeding
Acantharia feed on all kinds of small particulate materials, including bacteria, micro and picoplanktonic algae (diatoms and others), tintinnids and other ciliates and sarcodines, dinoflagellates, copepod nauplii, copepodids and adults, pelagic molluscs, etc. (Caron and Swanberg, 1990).
Many species contain symbiotic algae generally known as zooxanthellae, located either in the endoplasm or the ectoplasm. In natural assemblages of Acantharia, approximately half of the individuals have symbionts and the smallest and largest size classes have the lowest proportions of symbionts (Michaels 1988a, 1988b, 1991). Symbiont numbers appear to increase with size in the trophont stage, and they seem to be shed or consumed immediately prior to gametogenesis. Acantharian symbionts include dinoflagellates and prymnesiophytes, and some species have more than one symbiont type within the same host. Symbiont abundances vary within a host from a few large dinoflagellates, to many thousands of smaller cells. Most Acantharia have symbionts at some stage in their life cycle (Michaels, 1991). Symbiont reproduction is likely under host control. Most of the symbiont-fixed carbon is probably transferred to the host, suggesting that symbiotic algae are an important part of the nutrition of Acantharia as the organic carbon they produce can sometimes meet or exceed the inferred daily metabolic requirements of the host (Michaels, 1988a, 1988b, 1991; Caron et al., 1995). It is likely that nitrogen and phosphorus are supplied to the symbionts by the host.
Biogeochemistry
Acantharians play an important role in the budgets of strontium and other elements in the oceans. Mackenzie (1964) was the first to observe a significant increase in strontium to chlorinity ratios (Sr/Cl) at water depths of 500 to 800 meters, which he attributed to possible reaction with organic aggregates. A decade later, significantly different Sr/Cl ratios in shallow waters from the eastern North Pacific than those from deeper waters, as well as in the Pacific vs. the Atlantic oceans were described, suggesting that acantharians may be responsible for these trends (Brass and Turekian, 1974). To the extent that acantharians affect seawater Sr/Cl ratios, the trace metals reported in their skeletons and cysts suggests that cycling of other oceanic elements, like Ba and Ca, may also be influenced (Bernstein et al., 1998; De Deckker, 2004; van Beek et al., 2009).
Distribution
Acantharia are common in surface tropical and subtropical oceanic waters worldwide. In temperate and polar seas, as well as in nearshore areas, they occur in much lower numbers. Reported acantharian abundances vary widely, from <1 to 50 ind. l-1 (Caron and Swanberg, 1990). They are generally restricted to the illuminated upper layer of the ocean. On calm days Acantharia usually aggregate in the upper few meters of the water column; this surface affinity is likely related to the photosynthetic physiology of their symbiotic algae. However, upon wind or rain disturbance, they swiftly descend to lower strata, returning to the surface once adequate conditions there have reestablished (Schewiakoff, 1926).
Available information suggests that acantharians are geographically (but not ecologically) cosmopolitan. Almost all species have tropical and/or subtropical distribution (Reshetnjak, 1981). The number species that dwell in temperate-cold waters is comparatively low, probably around 10-20, and most of their records in colder waters are associated with the influences of warm-water currents.
Taxonomy
Acantharia comprises around 50 genera and 150 species. The primary reference for the systematics is the monograph by Schewiakoff (1926), who provided remarkable drawings and a detailed description of acantharian taxonomy. Schewiakoff's system takes the character of the cell body into account, whereas his predecessors used skeletal morphology almost exclusively for taxonomic discrimination. More recently, general descriptions of morphology and systematics, in some cases largely compiled from Schewiakoff (1926), were produced by Trégouboff (1953), Reshetnjak (1981), Cachon and Cachon (1985), Febvre (1990), Bernstein et al. (1999), and Febvre et al. (2000). Reshetnjak's (1981) monograph is particularly relevant since it reviews all known acantharian species, including distributional data, identification keys, and diagnoses. The ecology of Acantharia has been discussed in several articles covering their abundance and distribution, carbon cycling, etc. (Beers et al., 1975, 1982; Bishop et al., 1977, 1978, 1980; Massera Bottazzi and Andreoli, 1974, 1982a, 1982b; Michaels, 1988a, 1988b, 1991; Michaels et al., 1995; Caron et al., 1995; Caron and Swanberg, 1990).
References
Amaral Zettler, L., Sogin M.L. and Caron D.A. 1997. Phylogenetic relationships between the Acantharea and the Polycystinea: A molecular perspective on Haeckel’s Radiolaria. Proceedings of the National Academy of Sciences of the US, 94:11411–11416.
Beers, J.R., Reid, F.M.H. and Stewart G.L. 1975. Microplankton of the North Pacific Central Gyre. Population structure and abundance, June 1973. Internationale Revue der Gesamten Hydrobiologie, 60:607-638.
Beers, J.R., Reid, F.M.H. and Stewart, G.L. 1982. Seasonal abundance of the microplankton population in the North Pacific central gyre. Deep-Sea Research, 29:227-245.
Bernstein, R., Kling, S.A. and Boltovskoy, D. 1999. Acantharia. South Atlantic Zooplankton, Boltovskoy, D. [ed.], Backhuys Publishers, Leiden, The Netherlands, 75-147.
Bernstein, R. E., Byrne, R. H. and Schijf, J. 1998. Acantharians: a missing link in the oceanic biogeochemistry of barium. Deep-Sea Research, 45:491–505.
Bishop, J.K.B., Collier, R.W., Ketten, D.R. and Edmond, J.M. 1980. The chemistry, biology and vertical flux of particulate matter from the upper 400 m of the Panama Basin. Deep-Sea Reseach, 27:615-640.
Bishop, J.K.B., Edmond, J.M., Ketten, D.R., Bacon, M.P. and Silker, W.B. 1977. The chemistry, biology and vertical flux of particulate matter from the upper 400 m of the equatorial Atlantic Ocean. Deep-Sea Research, 24:511-548.
Bishop, J.K.B., Ketten, D.R. and Edmond, J.M. 1978. The chemistry, biology and vertical flux of particulate matter from the upper 400 m of the Cape Basin in the southeast Atlantic Ocean. Deep-Sea Research, 25:1121-1161.
Brass, G.W. and Turekian, K.K. 1974. Strontium distribution in GEOSECS oceanic profiles. Earth and Planetary Science Letters, 23:141-148.
Cachon, J. and Cachon, M. 1985. Superclass Actinopoda, Calkins 1902. Class Acantharea. An illustrated guide to the Protozoa, Society of Protozoologists, Lawrence, USA, 274-283.
Caron, D.A., Michaels, A.F., Swanberg, N.R. and Howse, F.A. 1995. Primary productivity by symbiont-bearing planktonic sarcodines (Acantharia, Radiolaria and Foraminifera) in surface waters near Bermuda. Journal of Plankton Research, 17:103-129.
Caron, D.A. and Swanberg, N.R. 1990. The ecology of planktonic sarcodines. Reviews in Aquatic Sciences, 3:147-180.
Chow, T.J. and Thompson, T.C. 1955. Flame photometric determination of strontium in sea water. Analitical Chemistry, 27:18-21.
De Deckker, P. 2004. On the celestite-secreting Acantharia and their effect on seawater strontium to calcium ratios. Hydrobiologia, 517:1–13.
Febvre, J. 1990. Phylum Actinopoda. Class Acantharia. Handbook of Protoctista, Margulis, L., Corliss, J.O., Melkonian, M. and Chapman, D.J., [eds.], Jones & Bartlett Publishers, Boston, USA, 363-379.
Febvre, C., Febvre, J. and Michaels, A. 2000. Subphylum Acantharia Haeckel, 1881. An illustrated guide to the Protozoa, Lee, J. [ed.], Second Edition, Society of Protozoologists, Lawrence, Kansas, USA, pp. 994-1022.
Gilga, I.C., Amaral-Zettler, L.A., Countwaya, P.D., Moorthic, S., Schnetzera, A. and Caron D.A. 2010. Phylogenetic affiliations of mesopelagic Acantharia and acantharian-like environmental 18S rRNA genes off the Southern California Coast. Protist, 161:197-211.
Haeckel, E. 1887. Report on Radiolaria collected by H.M.S. Challenger during the years 1873-1876. Rep. Sci. Res. Voyage H.M.S. Challenger 1873-76, 18:1-1803. d
López-García, P., Rodríguez -Valera, F., Moreira, D. 2002. Toward the monophyly of Haeckel’s Radiolaria: 18s rRNA environmental data support the sisterhood of Polycystinea and Acantharea. Molecular Biologt and Evolution, 19:118-121.
Mackenzie, F. 1964. Strontium content and variable strontium-chlorinity relationship of Sargasso sea water. Science, 146:517-518.
Massera Bottazzi, E. and Andreoli, M.G. 1974. Distribution of Acantharia in the North Atlantic. Archives for Oceanography and Limnology, 18:115-145.
Massera Bottazzi, E. and Andreoli, M.G. 1982a. Distribution of adult and juvenile Acantharia (Protozoa Sarcodina) in the Atlantic Ocean. Journal of Plankton Research, 4:757-777.
Massera Bottazzi, E. and Andreoli, M.G. 1982b. Distribution of the Acantharia in the Western Sargasso Sea in correspondence with "thermal fronts". Journal of Protozoology, 29:162-169.
Michaels, A.F., Caron, D.A., Swanberg, N.R., Howse, F.A. and Michaels C.M. 1995. Planktonic sarcodines (Acantharia, Radiolaria and Foraminifera) in surface waters near Bermuda: Abundance, biomass and vertical flux. Journal of Plankton Research, 17:131-163.
Michaels, A.F. 1988a. Vertical distribution and abundance of Acantharia and their symbionts. Marine Biology, 97:559-569.
Michaels, A.F. 1988b. Acantharia in the carbon and nitrogen cycles of the Pacific Ocean. Ph. D. Diss., Univ. California, Santa Cruz.
Michaels, A.F. 1991. Acantharian abundance and symbiont productivity at the VERTEX seasonal station. Journal of Plankton Research, 13:399-418.
Müller, J. 1859. Einige neue Polycysten und Acanthometren des Mittelmeers. Physic abhandlungen der Königlicher Akademie der Wissenschaften aus der Jahre 1858, Berlin, pp. 1-62.
Polet, S., Berney, C., Fahrni, J. and Pawlowski, J. 2004. Small-subunit ribosomal RNA gene sequences of Phaeodarea challenge the monophyly of Haeckel's Radiolaria. Protist, 155:53-63.
Reshetnjak, V.V. 1981. Akantarii. Fauna SSSR, 123, Akad. Nauk SSSR, Zool. Inst., Nauka, Leningrad, pp. 1-210.
Schewiakoff, W. 1926. Die Acantharia des Golfes von Neapel. Fauna Flora Golfo Napoli, Monogr. 37:1-755.
Trégouboff, G. 1953. Classe des Acanthaires. Traité de Zoologie, P.P. Grassé [ed.), Masson, Paris, 271-320.
van Beek, P., Sternberg, E., Reyss, J.-L., Souhaut, M., Robin, E. and Jeandel, C. 2009. 228Ra/226Ra and 226Ra/Ba ratios in the Western Mediterranean Sea: Barite formation and transport in the water column. Geochimica et Cosmochimica Acta, 73:4720-4737.
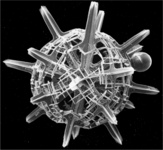
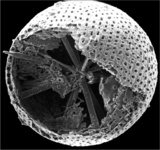
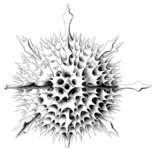
Title Illustrations

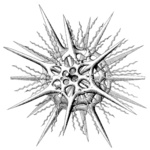
| Scientific Name | Phatnacantha icosaspis |
|---|---|
| Reference | Bernstein, R., Kling, S.A. and Boltovskoy, D. 1999. Acantharia. South Atlantic Zooplankton, Boltovskoy, D. [ed.], Backhuys Publishers, Leiden, The Netherlands, 75-147. |
| Creator | Bernstein et al. 1999 |
| Specimen Condition | Dead Specimen |
| Identified By | R, Bernstein |
| Copyright | © |
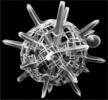
| Scientific Name | Amphibelone anomala |
|---|---|
| Reference | Bernstein, R., Kling, S.A. and Boltovskoy, D. 1999. Acantharia. South Atlantic Zooplankton, Boltovskoy, D. [ed.], Backhuys Publishers, Leiden, The Netherlands, 75-147. |
| Creator | Bernstein et al. 1999 |
| Specimen Condition | Dead Specimen |
| Identified By | R. Bernstein |
| Copyright | © |
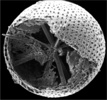
| Scientific Name | Acantharia |
|---|---|
| Reference | Bernstein, R., Kling, S.A. and Boltovskoy, D. 1999. Acantharia. South Atlantic Zooplankton, Boltovskoy, D. [ed.], Backhuys Publishers, Leiden, The Netherlands, 75-147. |
| Creator | Bernstein et al. 1999 |
| Specimen Condition | Dead Specimen |
| Identified By | R, Bernstein |
| Copyright | © |
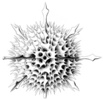
| Scientific Name | Aconthaspis hastata |
|---|---|
| Reference | Haeckel, E., 1887. Report on the Radiolaria collected by the H.M.S. Challenger during the Years 1873-1876. Report on the Scientific Results of the Voyage of the H.M.S. Challenger, Zoology 18: 1-1803. |
| Creator | Haeckel (1887) |
| Identified By | Haeckel (1887) |
| Scientific Name | Lychnaspis polyancistra |
|---|---|
| Reference | Haeckel, E., 1887. Report on the Radiolaria collected by the H.M.S. Challenger during the Years 1873-1876. Report on the Scientific Results of the Voyage of the H.M.S. Challenger, Zoology 18: 1-1803. |
| Creator | Haeckel (1887) |
| Identified By | Haeckel (1887) |
| Scientific Name | Hexacolpus infundibulum |
|---|---|
| Reference | Haeckel, E., 1887. Report on the Radiolaria collected by the H.M.S. Challenger during the Years 1873-1876. Report on the Scientific Results of the Voyage of the H.M.S. Challenger, Zoology 18: 1-1803. |
| Creator | Haeckel (1887) |
| Identified By | Haeckel (1887) |
About This Page
Note: This contribution is largely based on the review chapter “Acantharia”, by R. Bernstein, S.A. Kling and D. Boltovskoy, published in 1999 in the book “South Atlantic Zooplankton” (D. Boltovskoy, ed.), Backhuys Publishers, Leiden.
Nancy M. Correa

Servicio de Hidrografía Naval
Correspondence regarding this page should be directed to Demetrio Boltovskoy at
Page copyright © 2010 and Nancy M. Correa
 Page: Tree of Life
Acantharia.
Authored by
Demetrio Boltovskoy and Nancy M. Correa.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Acantharia.
Authored by
Demetrio Boltovskoy and Nancy M. Correa.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 28 September 2010
- Content changed 28 September 2010
Citing this page:
Boltovskoy, Demetrio and Nancy M. Correa. 2010. Acantharia. Version 28 September 2010 (under construction). http://tolweb.org/Acantharia/2385/2010.09.28 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site